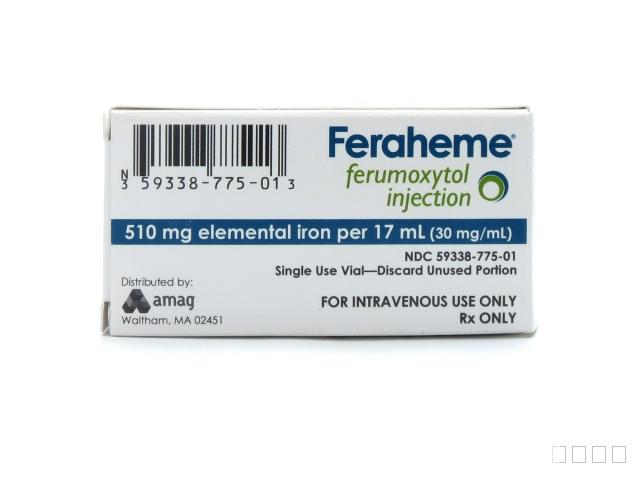
NDC:59338-775-01活性物质:FERUMOXYTOL剂量:510 mg/ 17 mL服用方式:静脉注射NDA:NDA022180 首次上市时间:07/13/2009分销商:AMAG Pharmaceuticals, Inc.生产商一:Baxter Pharmaceutical Solutions生产商二:Patheon Manufacturing Services LLC说明书:PDF
药品名称:Revolade 25 mg 14 tab药品简介:治疗适应症Revolade适用于自诊断后持续6个月或更长时间且对其他治疗(例如皮质类固醇,免疫球蛋白)无效的1岁及以上的原发性免疫性血小板减少症(ITP)的患者的治疗。在患有慢性丙型肝炎病毒(HCV)感染的成年患者中,应发生革命性反应,以治疗血小板减少症,其中血小板减少症的程度是阻止其开始或限制维持最佳干扰素疗法的主要因素。在患有获得性严重再生障碍性贫血(SAA)的成年患者中出现了恶变,这些患者要么对先前的免疫抑制治疗无效,要么经过严格的预处理,不适合进行造血干细胞移植药品通用名:Eltrombopag厂商:NOVARTIS剂型:片...
产品名称:SOGROYA上市时间:04/28/2023NDA/ANDA/BLA:761156持证商:NOVO NORDISK INCNDC:0169-2030-11,0169-2030-90,0169-2035-11,0169-2035-90,0169-2037-11,0169-2037-90说明书:https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761156s000lbl.pdf批准信:https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2020/761156Or...
产品名称:LIBTAYO上市时间:04/28/2023NDA/ANDA/BLA:761097持证商:REGENERON PHARMACEUTICALSNDC:61755-008-01说明书:https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761097s000lbl.pdf批准信:https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/761097Orig1s000ltr.pdf
产品名称:LIBTAYO上市时间:04/28/2023NDA/ANDA/BLA:761097持证商:REGENERON PHARMACEUTICALSNDC:61755-008-01说明书:https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761097s000lbl.pdf批准信:https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/761097Orig1s000ltr.pdf
产品名称:ESTRADIOL VALERATE上市时间:04/28/2023NDA/ANDA/BLA:216656持证商:XIROMEDNDC:70700-273-22,70700-274-22,70700-275-22说明书:https://dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=2e5c7559-7420-168d-6272-92bb4d20035d&type=pdf批准信:
产品名称:SYMBICORT AEROSPHERE上市时间:04/28/2023NDA/ANDA/BLA:216579持证商:ASTRAZENECANDC:0310-2009-12说明书:https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216579s000lbl.pdf批准信:
产品名称:VIGABATRIN上市时间:04/28/2023NDA/ANDA/BLA:215519持证商:PROPEL PHARMANDC:说明书:批准信:
产品名称:DOLUTEGRAVIR上市时间:04/28/2023NDA/ANDA/BLA:215319持证商:LAURUS GENERICS INCNDC:说明书:批准信:https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/215319Orig1s000TAltr.pdf
产品名称:LIQREV上市时间:04/28/2023NDA/ANDA/BLA:214952持证商:CMP DEV LLCNDC:46287-055-01说明书:https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/214952s000lbl.pdf批准信:https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/214952Orig1s000ltr.pdf
产品名称:VEKLURY上市时间:04/28/2023NDA/ANDA/BLA:214787持证商:GILEAD SCIENCES INCNDC:61958-2902-2,61958-2901-2说明书:https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214787Orig1s000lbl.pdf批准信:https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2020/214787Orig1s000ltr.pdf
产品名称:DEFERASIROX上市时间:04/28/2023NDA/ANDA/BLA:214559持证商:AUCTANDC:42806-371-30,42806-372-30,42806-373-30,72647-371-30,72647-372-30,72647-373-30说明书:批准信: