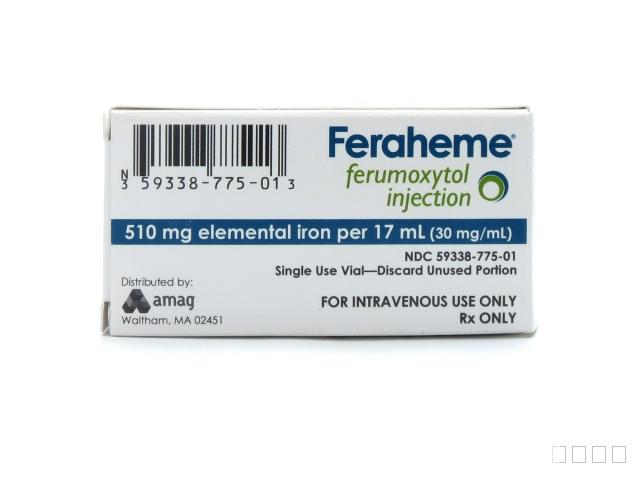
NDC:59338-775-01活性物质:FERUMOXYTOL剂量:510 mg/ 17 mL服用方式:静脉注射NDA:NDA022180 首次上市时间:07/13/2009分销商:AMAG Pharmaceuticals, Inc.生产商一:Baxter Pharmaceutical Solutions生产商二:Patheon Manufacturing Services LLC说明书:PDF
药品名: ABILIFY MAINTENA KIT (ARIPIPRAZOLE) ANDA: NDA #202971 NDC:59148-019-71 制造公司: OTSUKA PHARM CO LTD 活性物质: ARIPIPRAZOLE 剂量: 400MG/VIAL 使用方式: FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR 营销状态: Prescription 批准日期: 10/08/2014批准类型: Approval ...
产品名称:SOGROYA上市时间:04/28/2023NDA/ANDA/BLA:761156持证商:NOVO NORDISK INCNDC:0169-2030-11,0169-2030-90,0169-2035-11,0169-2035-90,0169-2037-11,0169-2037-90说明书:https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761156s000lbl.pdf批准信:https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2020/761156Or...
产品名称:LIBTAYO上市时间:04/28/2023NDA/ANDA/BLA:761097持证商:REGENERON PHARMACEUTICALSNDC:61755-008-01说明书:https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761097s000lbl.pdf批准信:https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/761097Orig1s000ltr.pdf
产品名称:LIBTAYO上市时间:04/28/2023NDA/ANDA/BLA:761097持证商:REGENERON PHARMACEUTICALSNDC:61755-008-01说明书:https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761097s000lbl.pdf批准信:https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/761097Orig1s000ltr.pdf
产品名称:ESTRADIOL VALERATE上市时间:04/28/2023NDA/ANDA/BLA:216656持证商:XIROMEDNDC:70700-273-22,70700-274-22,70700-275-22说明书:https://dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=2e5c7559-7420-168d-6272-92bb4d20035d&type=pdf批准信:
产品名称:SYMBICORT AEROSPHERE上市时间:04/28/2023NDA/ANDA/BLA:216579持证商:ASTRAZENECANDC:0310-2009-12说明书:https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216579s000lbl.pdf批准信:
产品名称:VIGABATRIN上市时间:04/28/2023NDA/ANDA/BLA:215519持证商:PROPEL PHARMANDC:说明书:批准信:
产品名称:DOLUTEGRAVIR上市时间:04/28/2023NDA/ANDA/BLA:215319持证商:LAURUS GENERICS INCNDC:说明书:批准信:https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/215319Orig1s000TAltr.pdf
产品名称:LIQREV上市时间:04/28/2023NDA/ANDA/BLA:214952持证商:CMP DEV LLCNDC:46287-055-01说明书:https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/214952s000lbl.pdf批准信:https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/214952Orig1s000ltr.pdf
产品名称:VEKLURY上市时间:04/28/2023NDA/ANDA/BLA:214787持证商:GILEAD SCIENCES INCNDC:61958-2902-2,61958-2901-2说明书:https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214787Orig1s000lbl.pdf批准信:https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2020/214787Orig1s000ltr.pdf
产品名称:DEFERASIROX上市时间:04/28/2023NDA/ANDA/BLA:214559持证商:AUCTANDC:42806-371-30,42806-372-30,42806-373-30,72647-371-30,72647-372-30,72647-373-30说明书:批准信: