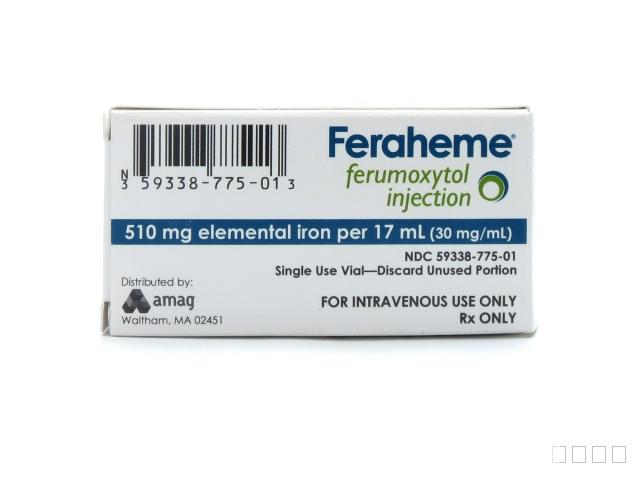
NDC:59338-775-01活性物质:FERUMOXYTOL剂量:510 mg/ 17 mL服用方式:静脉注射NDA:NDA022180 首次上市时间:07/13/2009分销商:AMAG Pharmaceuticals, Inc.生产商一:Baxter Pharmaceutical Solutions生产商二:Patheon Manufacturing Services LLC说明书:PDF
药品编号 06697999 持证商 SCHAPER & BRÜMMER GmbH & Co. KG 包装规格 100 St Packungsnorm N3 产品名 Esberitox Tabletten 剂型 Tabletten Pfl. Arzneimittel ja 需要医生开处方 nein Apothekenpflichtig ja 信息来源:https://www.besamex.de/produkt/esberitox-tabletten-100-st-06697999.html
医薬品情報総称名ベネクレクスタ一般名ベネトクラクス欧文一般名Venetoclax製剤名ベネトクラクス錠薬効分類名抗悪性腫瘍剤BCL-2阻害剤薬効分類番号4291ATCコードL01XX52KEGG DRUGD10679 ベネトクラクス商品一覧 米国の商品 相互作用情報JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2021年3月 改訂(効能又は効果、用法及び用量変更)(第5版)1.警告 2.禁忌 4.効能または効果&nbs...
医薬品情報総称名コララン一般名イバブラジン塩酸塩欧文一般名Ivabradine Hydrochloride製剤名イバブラジン塩酸塩錠薬効分類名HCNチャネル遮断薬薬効分類番号2190ATCコードC01EB17KEGG DRUGD08095 イバブラジン塩酸塩商品一覧 米国の商品 相互作用情報JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2023年2月 改訂(第5版)2.禁忌 4.効能または効果 6.用法及び用量&nbs...
医薬品情報総称名ブリニューラ一般名セルリポナーゼ アルファ(遺伝子組換え)欧文一般名Cerliponase Alfa(Genetical Recombination)製剤名セルリポナーゼ アルファ(遺伝子組換え)製剤薬効分類名セロイドリポフスチン症2型治療剤薬効分類番号3959ATCコードA16AB17KEGG DRUGD10813 セルリポナーゼアルファ商品一覧 米国の商品JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2022年8月 改訂(第4版...
医薬品情報総称名クリースビータ一般名ブロスマブ(遺伝子組換え)欧文一般名Burosumab(Genetical Recombination)製剤名ブロスマブ(遺伝子組換え)製剤薬効分類名ヒト型抗FGF23モノクローナル抗体薬効分類番号3999ATCコードM05BX05KEGG DRUGD10913 ブロスマブ商品一覧 米国の商品 相互作用情報JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2021年12月 改訂(第3版)2.禁忌 ...
医薬品情報総称名ポートラーザ一般名ネシツムマブ(遺伝子組換え)欧文一般名Necitumumab(Genetical Recombination)製剤名ネシツムマブ(遺伝子組換え)注射液薬効分類名抗悪性腫瘍剤 ヒト型抗EGFR注1)モノクローナル抗体 注1)EGFR:Epidermal Growth Factor Receptor(上皮細胞増殖因子受容体)薬効分類番号4291ATCコードL01FE03KEGG DRUGD10018 ネシツムマブ商品一覧 米国の商品KEGG DGROUPDG03162 EGFR阻害薬商品一覧JAPIC添付文書(PDF)この情報は&nb...
医薬品情報総称名ヴァンフリタ一般名キザルチニブ塩酸塩欧文一般名Quizartinib Hydrochloride製剤名キザルチニブ塩酸塩錠薬効分類名抗悪性腫瘍剤FLT3阻害剤薬効分類番号4291ATCコードL01EX11KEGG DRUGD09956 キザルチニブ塩酸塩商品一覧 相互作用情報JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2020年7月 改訂(第3版)1.警告 2.禁忌 4.効能または効果 6.用法及び用...
医薬品情報総称名ロズリートレク一般名エヌトレクチニブ欧文一般名Entrectinib製剤名エヌトレクチニブカプセル薬効分類名抗悪性腫瘍剤チロシンキナーゼ阻害剤薬効分類番号4291ATCコードL01EX14KEGG DRUGD10926 エヌトレクチニブ商品一覧 米国の商品 相互作用情報JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2022年8月 改訂(第8版)1.警告 2.禁忌 4.効能または効果 6.用法及...
医薬品情報総称名ユルトミリス一般名ラブリズマブ(遺伝子組換え)欧文一般名Ravulizumab(Genetical Recombination)製剤名ラブリズマブ(遺伝子組換え)点滴静注製剤Ravulizumab(Genetical Recombination)薬効分類名抗補体(C5)モノクローナル抗体製剤薬効分類番号6399KEGG DRUGD11054 ラブリズマブ商品一覧 米国の商品 相互作用情報JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添...
医薬品情報総称名デファイテリオ一般名デフィブロチドナトリウム欧文一般名Defibrotide Sodium製剤名デフィブロチドナトリウム静注薬効分類名肝類洞閉塞症候群治療剤薬効分類番号3919ATCコードB01AX01KEGG DRUGD07423 デフィブロチドナトリウム商品一覧 米国の商品 相互作用情報JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2019年6月 作成(第1版)1.警告 2.禁忌 4.効能または効果...
医薬品情報総称名オンパットロ一般名パチシランナトリウム製剤名パチシランナトリウム注射液薬効分類名トランスサイレチン型アミロイドーシス治療薬薬効分類番号1290ATCコードN07XX12KEGG DRUGD10794 パチシランナトリウム商品一覧 米国の商品JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2021年2月 改訂(第2版)2.禁忌 4.効能または効果 6.用法及び用量 8.重要な基本的注意 11.副作...