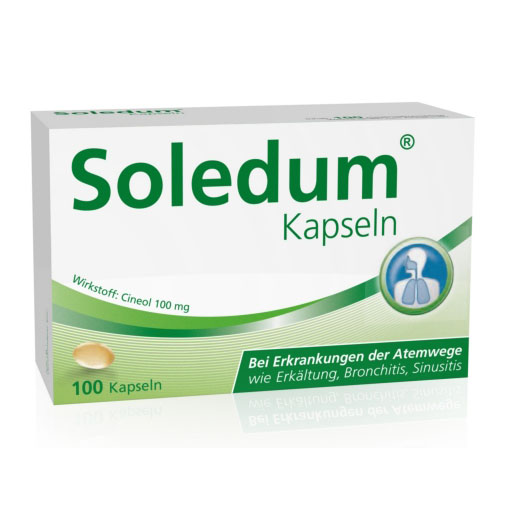
药品编号 06349838 持证商 MCM KLOSTERFRAU Vertr. GmbH 包装规格 100 St Packungsnorm N3 产品名 Soledum Kapseln 100mg 剂型 Kapseln magensaftresistent Monopräparat ja 活性物质 Cineol 需要医生开处方 nein Apothekenpflichtig ja 信息来源:https://www.besamex.de/produkt/soledum-100-mg-magensaftresistente-kapseln-100-st-06349838.html
药品编号 06697999 持证商 SCHAPER & BRÜMMER GmbH & Co. KG 包装规格 100 St Packungsnorm N3 产品名 Esberitox Tabletten 剂型 Tabletten Pfl. Arzneimittel ja 需要医生开处方 nein Apothekenpflichtig ja 信息来源:https://www.besamex.de/produkt/esberitox-tabletten-100-st-06697999.html
医薬品情報総称名ノクサフィル一般名ポサコナゾール欧文一般名Posaconazole製剤名ポサコナゾール錠薬効分類名深在性真菌症治療剤薬効分類番号6179ATCコードJ02AC04KEGG DRUGD02555 ポサコナゾール商品一覧 米国の商品 相互作用情報KEGG DGROUPDG01523 トリアゾール系抗真菌薬商品一覧JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2022年7月 改訂(第6版)2.禁忌 4.効能ま...
医薬品情報総称名コレクチム一般名デルゴシチニブ欧文一般名Delgocitinib製剤名デルゴシチニブ軟膏薬効分類名外用ヤヌスキナーゼ(JAK)阻害剤薬効分類番号2699KEGG DRUGD11046 デルゴシチニブ商品一覧KEGG DGROUPDG02020 JAK阻害薬商品一覧JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2023年1月 改訂(第6版)2.禁忌 4.効能または効果 6.用法及び用量 11.副作用&nb...
医薬品情報総称名ユリス一般名ドチヌラド欧文一般名Dotinurad製剤名ドチヌラド薬効分類名選択的尿酸再吸収阻害薬−高尿酸血症治療剤−薬効分類番号3949KEGG DRUGD11071 ドチヌラド商品一覧 相互作用情報JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2022年11月 改訂(第5版)2.禁忌 4.効能または効果 6.用法及び用量 8.重要な基本的注意 10.相互作用 11.副作用&nbs...
医薬品情報総称名アネレム一般名レミマゾラムベシル酸塩欧文一般名Remimazolam Besilate製剤名注射用レミマゾラムべシル酸塩薬効分類名全身麻酔剤薬効分類番号1119ATCコードN05CD14KEGG DRUGD10194 レミマゾラムベシル酸塩商品一覧 米国の商品 相互作用情報JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2020年8月 改訂(第2版)2.禁忌 4.効能または効果 6.用法及び用量 ...
医薬品情報総称名ピフェルトロ一般名ドラビリン欧文一般名Doravirine製剤名ドラビリン錠薬効分類名抗ウイルス化学療法剤薬効分類番号6250ATCコードJ05AG06KEGG DRUGD10624 ドラビリン商品一覧 米国の商品 相互作用情報KEGG DGROUPDG03107 抗HIV薬商品一覧JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2022年3月 改訂(第3版)2.禁忌 4.効能または効果 6.用...
医薬品情報総称名ラスビック一般名ラスクフロキサシン塩酸塩欧文一般名Lascufloxacin Hydrochloride製剤名ラスクフロキサシン塩酸塩錠薬効分類名ニューキノロン系経口抗菌剤薬効分類番号6241ATCコードJ01MA25KEGG DRUGD10772 ラスクフロキサシン塩酸塩商品一覧 相互作用情報KEGG DGROUPDG01549 ニューキノロン系抗生物質商品一覧JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2020年12...
医薬品情報総称名トリンテリックス一般名ボルチオキセチン臭化水素酸塩欧文一般名Vortioxetine Hydrobromide製剤名ボルチオキセチン臭化水素酸塩錠薬効分類名セロトニン再取り込み阻害・セロトニン受容体調節剤薬効分類番号1179ATCコードN06AX26KEGG DRUGD10185 ボルチオキセチン臭化水素酸塩商品一覧 米国の商品 相互作用情報JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2021年12月 改訂(第2版)2....
医薬品情報総称名エベレンゾ一般名ロキサデュスタット欧文一般名Roxadustat製剤名ロキサデュスタット錠薬効分類名HIF-PH阻害薬腎性貧血治療薬薬効分類番号3999ATCコードB03XA05KEGG DRUGD10593 ロキサデュスタット商品一覧 相互作用情報JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2022年11月 改訂(第7版)1.警告 2.禁忌 4.効能または効果 6.用法及び用量 8.重要な...
医薬品情報総称名エクフィナ一般名サフィナミドメシル酸塩欧文一般名Safinamide Mesilate製剤名サフィナミドメシル酸塩錠薬効分類名パーキンソン病治療剤薬効分類番号1169ATCコードN04BD03KEGG DRUGD10191 サフィナミドメシル酸塩商品一覧 米国の商品 相互作用情報KEGG DGROUPDG01967 抗パーキンソン病薬商品一覧JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米の医薬品添付文書はこちらから検索することができます。添付文書情報2020年12月 改訂(第...
医薬品情報総称名イスパロクト一般名ツロクトコグ アルファ ペゴル(遺伝子組換え)欧文一般名Turoctocog Alfa Pegol(Genetical Recombination)製剤名ツロクトコグ アルファ ペゴル(遺伝子組換え)薬効分類名ペグ化遺伝子組換え型血液凝固第VIII因子製剤薬効分類番号6349ATCコードB02BD02KEGG DRUGD10758 ツロクトコグアルファペゴル商品一覧KEGG DGROUPDG00170 血液凝固第VIII因子商品一覧JAPIC添付文書(PDF)この情報は KEGG データベースにより提供されています。日米...